Source of Administrative boundaries: European Boundary Map (EBM) v9.1, EuroGeographics (2015);
The Global Administrative Unit Layers (GAUL) dataset, implemented by FAO within the CountrySTAT
and Agricultural Market Information System (AMIS) projects;
The data sets are slightly modified by UMG/SHIP (reduced number of points per polygon)
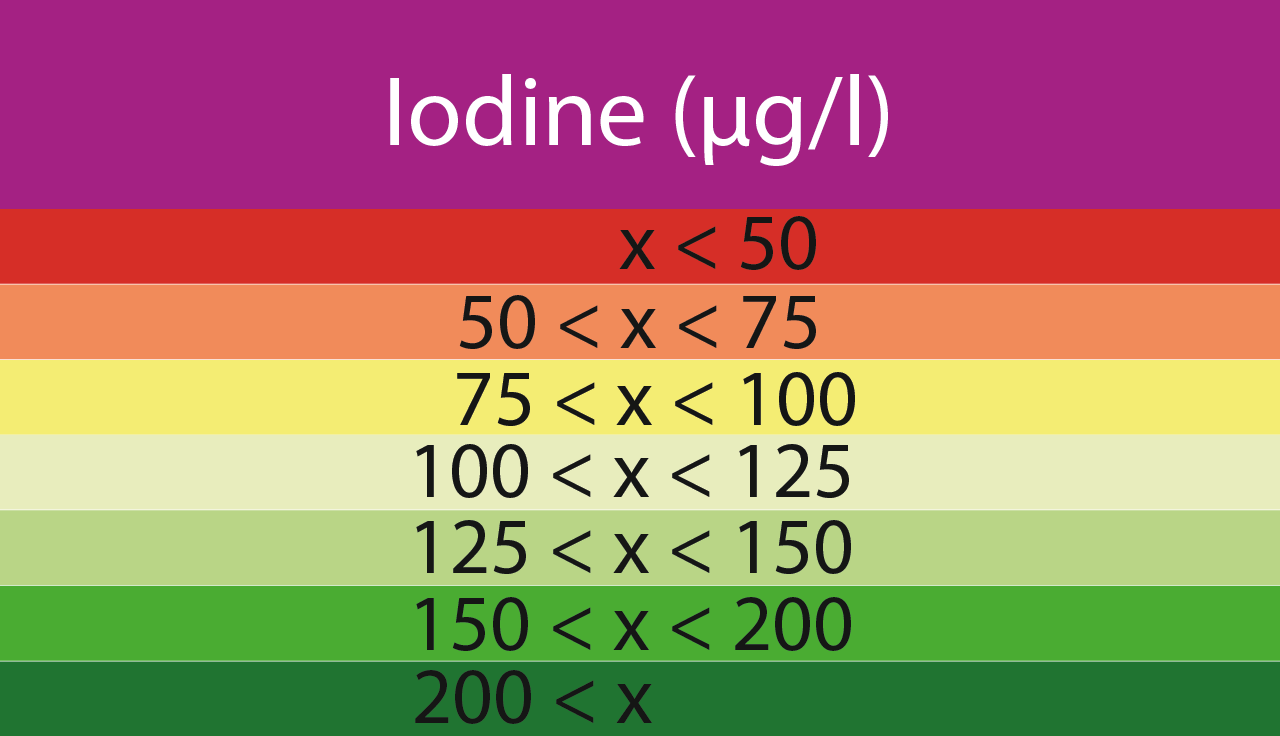
Iodine (µg/l)
x < 50
50 ≤ x < 75
75 ≤ x < 100
100 ≤ x < 125
125 ≤ x < 150
150 ≤ x < 200
200 ≤ x
Urinary iodine measurements were standardized in the EUthyroid Central Laboratory in Helsinki. For each study, 75 urine samples were re-measured in Helsinki and compared to the original data by Bland & Altman plots and linear regression models.
The Biochemistry laboratory of Genomics and Biomarkers unit at the National Institute for Health and Welfare (THL) in Helsinki (No.T077) has been accredited by Finnish Accreditation Service FINAS and it fulfills the requirement of the standard SFS-EN ISO/IEC 17025. The scope of accreditation covers the urinary iodine concentration method. Statistical analyses have been conducted by a senior statistician at Universitymedicine Greifswald (UMG).
Urinary iodine concentration measurements were carried out by inductively coupled plasma – mass spectrometry (ICP-MS) using an Agilent 7800 ICP-MS system. In brief, 100 µl of urine sample was extracted using basic solution. Iodine was scanned on m/z = 127 and tellurium was used as internal standard. The National Institute of Standards and Technology (NIST) reference standard materials SRM2670a (with certified mass concentration value) and SRM3668 Level 1 and Level 2 were used to ensure accuracy of urinary iodine determinations. The quality of test results is assured by participating in external quality assessment scheme Ensuring the Quality of Urinary Iodine Procedures (EQUIP).
Based on the linear regression models, formulas were derived for each lab comparison:
Iodine (Harmonized) = Intercept + Slope*Iodine (Original)
This transformation formula was applied to the original urinary iodine levels of the IDD monitoring studies to harmonize study data.
